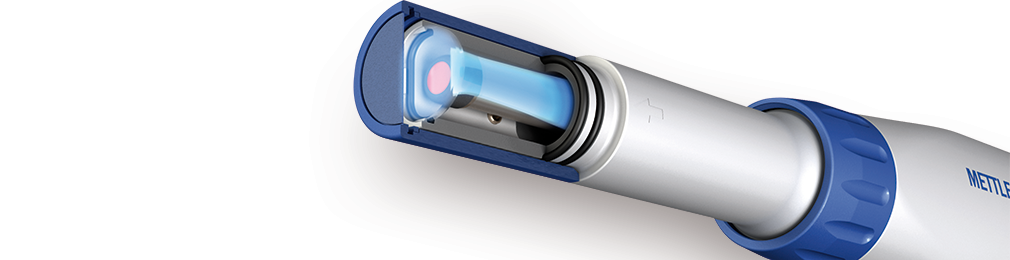
Dissolved Oxygen Electrode
Optical, Polarographic, and Galvanic Electrodes for Proper Determination of Dissolved Oxygen
A dissolved oxygen electrode determines how much oxygen is dissolved in a solution. As an indicator of quality, knowing the amount of free, non-compound oxygen in a product is important for many types of labs, including those involved in pharmaceutical research, food & beverage quality control, or environmental monitoring. METTLER TOLEDO manufactures optical, polarographic, and galvanic electrodes for accurate DO determinations in a wide range of laboratory and field applications.
Advantages of METTLER TOLEDO's Dissolved Oxygen Electrodes
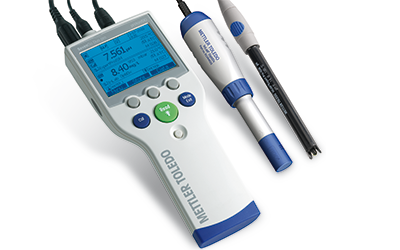
The Right Sensor for Your Needs
To determine oxygen levels accurately, reliable dissolved oxygen electrodes are needed. A combination of high-quality materials and effective technologies ensure that our optical, polarographic, and galvanic electrodes deliver accurate DO determinations in laboratory or field applications.

Optical Determinations
The InLab® OptiOx™ DO sensors use RDO® (Rugged Dissolved Oxygen) technology, simplifying your optical DO measurements. This means no sample oxygen is consumed during measurement, creating a fast, stable system that requires little maintenance. It is an excellent choice for BOD measurement applications (biological oxygen demand).

Polarographic Determinations
Designed for harsh environments and applications where optical measurements are not an option, METTLER TOLEDO’s polarographic dissolved oxygen electrodes are equipped with a reinforced fiberglass PPS shaft. These extremely robust DO electrodes also feature a highly permeable membrane to ensure accurate dissolved oxygen measurements.

Galvanic Determinations
A galvanic DO sensor contains two electrodes made of different metals (of different nobility) in an electrolyte solution. The electrodes are interconnected by wires to enable the flow of current. They are a suitable option to obtain quality measurements for the budget-minded and align perfectly with our Standard Meter line.
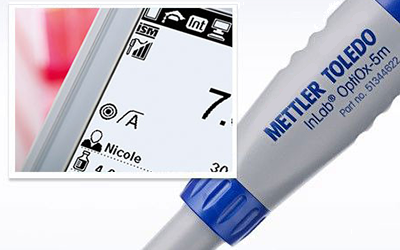
Quick and Traceable Results
Thanks to the Intelligent Sensor Management (ISM®) technology, the instrument automatically detects the connected DO sensor and uses the most up-to-date calibration data stored on it. This ensures safe, accurate, and traceable results.

Measure Safely in Harsh Environments
The dissolved oxygen electrodes from METTLER TOLEDO are rated IP67 to help ensure that the entire portable DO measuring system can withstand wet and demanding outdoor applications, while delivering accuracy and longevity.

All-In-One Solution
METTLER TOLEDO provides complete electrochemistry systems, from meters and sensors, to calibration solutions, and software. Benefit from the Intelligent Sensor Management (ISM®) technology to support data compliance.
Explore Our Services - Tailored to Fit Your Equipment
We support and service your measurement equipment through its entire life-cycle, from installation to preventive maintenance and calibration to equipment repair.
Support & Repair

FAQs
What types of electrodes can be used for measuring dissolved oxygen (DO)?
The following types of dissolved oxygen sensor technologies are available for laboratory and field applications:
a. Optical dissolved oxygen electrode (InLab OptiOx)
b. Polarographic dissolved oxygen electrode (InLab 605)
c. Galvanic dissolved oxygen electrode (LE621)
How does an optical dissolved oxygen electrode work?
An optical DO electrode uses a special dye embedded in a membrane at the sensor’s tip (as shown in the figure). This dye can be excited by absorbing blue light emitted internally by the sensor. As the excited dye returns to its ground state, it fluoresces by emitting red light, which is measured by a photodetector inside the sensor. When oxygen molecules are present on the membrane’s outer surface, they can absorb the excess energy of the excited dye. By doing so, they reduce (quench) the amount of fluorescence that reaches the photodetector. The more oxygen present in a sample, the more fluorescence quenching and the lower the measured signal. The sensor also contains a red light source. This light does not excite the dye and thus does not cause fluorescence but is merely reflected by the dye and measured by the photodetector. The red light is used as a reference to account for a decrease in the detected light that is not related to oxygen quenching, e.g., the decay of the dye or temperature-dependent sensitivity of the detector. For more detailed information, learn more in the following video.
 |
How does a polarographic DO electrode work?
The electrode has a silver anode surrounded by a noble metal cathode made from gold or platinum. These electrodes are polarized by a constant voltage provided by the instrument. Consequently, the anode acquires a positive charge, and the cathode a negative one. KCl is the electrolyte, and it is contained by a membrane that separates it from the sample. When oxygen enters the electrode, the oxygen molecules are reduced at the cathode to form hydroxide ions. Because the polarization potential is held constant, the oxygen reaction increases the electrical signal. This effect is proportional to the partial pressure of oxygen in the sample. The electrode uses a chemical reaction in which the silver anode is oxidized and consumed. In contrast, the cathode is noble and does not participate in the reaction. Instead, it provides a surface on which oxygen is reduced by electrons transported from the anode through the wire.
 |
How does a galvanic DO electrode work?
Containing two electrodes, the anode is usually made of zinc or lead, while the cathode is usually made of silver or another noble metal. The electrodes are interconnected by wires, allowing the current to flow between them. These components are encompassed in a shaft, which is sealed off by a membrane that is selectively permeable to oxygen (as shown in the figure). The electrolyte has to be aqueous and alkaline. The entry of oxygen into the electrode enables a chemical reaction in which the anode is oxidized (donates electrons) and consumed.
In contrast, the cathode is noble and does not participate in the reaction: it exists as a reaction surface onto which oxygen is reduced. The electrons transported from the anode to the cathode through the wire generate a current, which can be measured in the DO meter. The more oxygen that enters the system, the more current is generated.
 |
What are the differences between polarographic and galvanic dissolved oxygen electrodes?
Characteristic | Galvanic DO Electrode | Polarographic DO Electrode |
|
|
|
Therefore, galvanic sensors require no warm-up time and are more stable at a lower dissolved oxygen level than polarographic probes. On the contrary, polarographic sensors have a longer lifetime. For more information on the working principles of individual sensors, please refer to questions 3 and 4 above.
Is there any electrode preparation required for laboratory DO sensors before a measurement?
a. Electrochemical sensors must be checked for membrane integrity. Additionally, it must be ensured that the electrolyte is replenished correctly if electrolyte refilling is applicable.
b. When using a polarographic sensor, the proper polarization of the sensor needs to be ensured.
c. Optical laboratory DO sensors do not require any preparation prior to their usage.
Is it necessary to calibrate an optical dissolved oxygen electrode before performing measurements?
For standard oxygen measurements, a 1-point calibration at 100% oxygen saturation (water-saturated air) is sufficient for many applications. For low oxygen concentration measurements (below 10% or 0.8 mg/L), it is recommended to have a second calibration point using an oxygen-free standard solution (this corresponds to 0% oxygen saturation). For this purpose, zero oxygen tablets are dissolved in water to eliminate all the dissolved oxygen in it.
Is it necessary to stir the sample while measuring it with a laboratory DO sensor?
Stirring is necessary for the electrochemical laboratory DO sensors because the sensors consume oxygen while measuring. The stirring should be kept at a constant speed. In contrast to electrochemical sensors, optical DO electrodes do not require stirring because they do not consume oxygen. In order to reduce the measurement duration, the sensor tip should be submerged into the sample before starting the measurement. This procedure will allow the oxygen concentration and temperature to equilibrate. Air bubbles at the sensor tip must be avoided. Otherwise, the oxygen concentration of the air bubbles will also be measured, leading to false results.
 |
How should I store the laboratory DO sensors?
- General storage tips:
After a measurement, the sensor should be cleaned with water and wiped with a soft tissue. Especially when measuring biological samples, microbiological growth should be carefully avoided. For optimal performance, a sensor should be stored in a safe environment at temperatures between 5 and 45 °C; fast temperature changes should be avoided. - Galvanic DO sensor for laboratory applications:
For short-term storage, it should be rinsed with deionized water and placed in a storage solution. For long-term storage, it should also be short-circuited (to prevent deterioration due to continuous self-polarization) and stored in a cool place. - Polarographic DO sensor for laboratory applications:
During short-term storage, avoid the 6-hour polarization requirement; it can be left connected to the instrument. For long-term storage, it should be detached from the device because continuous polarization will gradually reduce its lifetime. Provided the sensor is filled with inner electrolyte and the protective cap is placed over the membrane, it can be stored for several months. However, to use the sensor again after more than three months of storage, the electrolyte should be replaced. If more than six months of storage is intended, the electrolyte should be removed. - Optical laboratory DO sensor:
An optical sensor should be stored dry. Sensors with a replaceable membrane module should have it exchanged as soon as the sensor shows signs of reduced performance.
Are METTLER TOLEDO's laboratory DO sensors waterproof?
Most are IP67 certified, ensuring that the entire portable system is able to withstand wet and demanding environments.
Can METTLER TOLEDO's laboratory DO probe measure temperature as well?
Most of our laboratory DO probes come with an integrated temperature probe that helps measure the correct temperature of a sample.
Can an InLab 605 laboratory DO sensor be used for field applications too?
Indeed, it is equipped with a glass fiber-reinforced PPS shaft and a measuring membrane protected by a steel mesh making this sensor optimal for demanding applications.
What is the Biological oxygen demand (BOD) and why is it necessary to measure BOD?
Biochemical oxygen demand (BOD) represents the amount of oxygen consumed by bacteria and other microorganisms while decomposing organic matter under aerobic conditions at a specified temperature. BOD is an important parameter in water treatment plants, indicating the degree of organic pollution in water. To learn more, you can refer to our guide dedicated to this topic: Biochemical Oxygen Demand From Theory to Practice. With the SevenExcellence DO meter setting up your own BOD determination process is possible in no time.
 |
Can a laboratory optical DO probe be used for measuring BOD as well?
Yes, the InLab OptiOx is perfectly equipped for measuring BOD. The special OptiOx BOD adapter makes the sensor perfectly suited for measurements in all standard BOD canisters.
Can the optical DO sensor only be used for lab applications?
No, the InLab OptiOx's robust design and matching accessories make it ideal for various applications, both in the laboratory and outdoors. The steel OptiOx protective guard (as shown below) offers sensor protection in hostile environments. It is light in weight, meaning it can be easily extended to lower measuring points.
 |








