Supersaturation is a non-equilibrium, physical state in which a solution contains more solute than the equilibrium solubility allows, given conditions such as temperature and pressure of the system. Supersaturation is also used to describe the level at which the solute concentration exceeds its solubility at given conditions, expressed as:
ΔC (supersaturation) = C (actual concentration) – C* (solubility).
What Is Supersaturation?
Why Is Supersaturation Important?
Supersaturation is important because it is the driving force for both crystal nucleation and growth. The control of supersaturation is key to attaining desired product attributes, especially final crystal size distribution and phase. Generally speaking, at low supersaturation, crystals can grow faster than they nucleate, resulting in a larger crystal size. At higher supersaturation, crystal nucleation dominates crystal growth, ultimately resulting in smaller crystals.
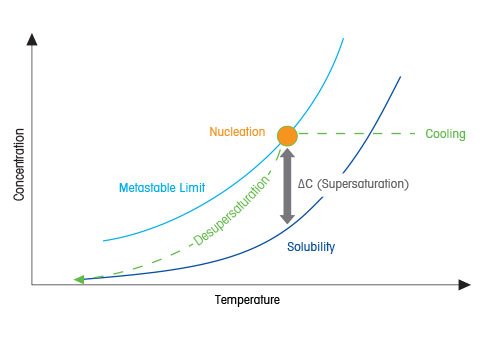
How Do You Control Supersaturation?
A typical way to generate supersaturation is to dissolve a substance in a solvent at elevated temperature and then cool the solution down. As the temperature is reduced, the system enters the metastable supersaturated state; as cooling continues, the metastable limit will be reached. At this point the nucleation process will occur, supersaturation will diminish or end and the liquid phase solute concentration will ultimately attain equilibrium at the solubility curve.
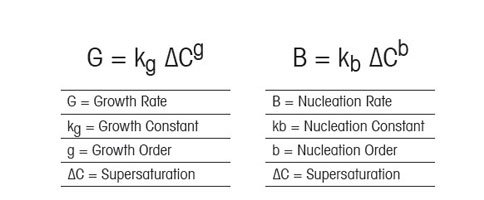
Crystal Nucleation Versus Growth
Nucleation is the birth of new crystal nuclei – either spontaneously from solution (primary nucleation) or in the presence of existing crystals (secondary nucleation). Crystal growth is the increase in size (or more accurately “characteristic length”) of crystals as solute is deposited from solution. The relationship between supersaturation, nucleation, and growth is defined by a well-known set of (somewhat simplified) equations first outlined by Nyvlt.
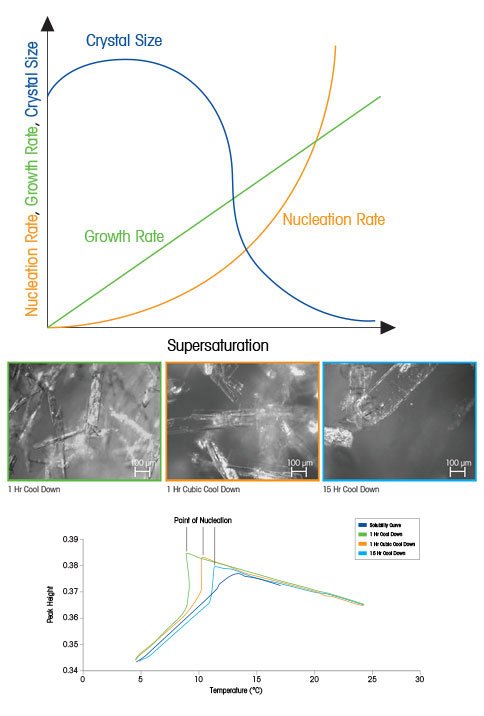
For organic crystallization systems, the value of the growth order (g) is typically between 1 and 2 and the value of the nucleation order (b) is typically between 5 and 10. When we plot these equations for a theoretical organic crystallization process the importance of supersaturation becomes clear. At low supersaturation, crystals can grow faster than they nucleate, resulting in a larger crystal size distribution. However, at higher supersaturation, crystal nucleation dominates crystal growth, ultimately resulting in smaller crystals. In the figure to the right, relating supersaturation to nucleation, growth, and crystal size clearly illustrate how controlling supersaturation is vitally important when it comes to creating crystals of the desired size and distribution.
Modern techniques, such as FTIR Spectroscopy with Attenuated Total Reflectance (ATR), allow solubility traces to be developed quickly and easily, and the prevailing level of supersaturation to be monitored continuously throughout a crystallization experiment. Faster cooling rates result in nucleation at lower temperatures and the highest level of supersaturation throughout the process. A very slow cool down results in a higher nucleation temperature and low supersaturation throughout the process. A one-hour cubic cool down (slow at first and fast at the end) has a medium level of supersaturation throughout. Higher supersaturation results in the smallest crystals – since nucleation will be favored over growth.
Technology For Supersaturation
METTLER TOLEDO offers a suite of Process Analytical Technologies to support crystallization research and development (R&D) and product manufacturing. These technologies can be used stand-alone or in combination with an automated chemical reactor, forming an integrated Crystallization Workstation. The technologies include:
- ReactIR – Inline, in-situ FTIR measures solution phase concentrations in real-time for solubility and supersaturation studies, and investigate kinetics and thermodynamics of polymorphism in solute-solvent crystallization studies.
- Raman Spectrometers - Inline ReactRaman tracks particle and crystallization formation in-situ as a function of time in solvent or slurry, and monitors crystal form changes as metastable polymorphs convert to thermodynamically stable forms.
- ParticleTrack – Focused Beam Reflectance Measurement (FBRM) is the industry-standard measurement technique used for in-process measurement of particles. A highly precise chord length distribution (CLD), sensitive to particle size and count, is reported in real time without the need for sampling or sample preparation.
- EasyViewer with iC Vision– Inline particle size analyzer provides real-time images of crystals, precipitates and particles. iC Vision provides particle image analysis for monitoring process changes with intuitive analytics, and quantifies particle size and shape with customizable algorithms.
Featured Article: Role of Kinetics and Thermodynamics in Reactive Crystallization of an API
ReactIR Investigates Kinetics and Mechanism of Polymorphism
Yun Cao, Shichao Du, Xiao Ke, Shijie Xu, Yangshan Lan, Teng Zhang, Weiwei Tang, Jingkang Wang, and Junbo Gong, “Interplay between Thermodynamics and Kinetics on Polymorphic Behavior of Vortioxetine Hydrobromide in Reactive Crystallization”, Org. Process Res. Dev, 2020, 24, 1233−1243.
Reactive crystallization is associated with elevated levels of supersaturation for the crystalline compounds formed by the chemical reaction. In this work, the authors investigated the effect of solvents and supersaturation on polymorphism in vortioxetine hydrobromide prepared by reactive crystallization. The experiments were performed in four different solvents with defined initial supersaturation, and the role of thermodynamics and kinetics on the formation and conversion of the two polymorphs were investigated. To better understand the mechanism of this reactive crystallization, in-situ IR (ReactIR) was applied.
Polymorph B was identified as the stable form, and the metastable polymorph A was preferred at high supersaturations in the four solvents tested. They also determined that polymorph A formed in solvents with low hydrogen bond acceptor ability. The ReactIR measurements showed that the effect of reaction kinetics on the polymorphism was not an issue. ReactIR measurements along with powder x-ray diffraction studies were also used to follow the reactive crystallization and polymorphic conversion. The researchers report that the nucleation and growth of form B were found to be the controlling step, and rate of conversion increases considerably with vortioxetine hydrobromide solubility and initial supersaturation.
Featured Article: Solvent Mediated Polymorphic Transformation
ReactIR and ParticleTrack Track Supersaturation and Stable Polymorph Crystal Growth
Dan Du, Guo-Bin Ren, Ming-Hui Qi, Zhong Li and Xiao-Yong Xu, “Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems”, Crystals, 2019, 9, 161.
The authors investigated polymorphism in the drug famoxadone and demonstrated the presence of 6 polymorphs. They undertook an investigation of solvent-mediated polymorphic transformation (SMPT) from metastable Form II to the stable Form I using in-situ PAT technology. ParticleTrack FBRM measurements measured chord length change and ReactIR with in situ probe monitored solution concentrations as a function of time. The SMPT process was performed in an EasyMax automated lab reactor system equipped with the aforementioned analytical probes.
They state that the famoxadone polymorphic transformation process has three stages consisting of metastable form dissolution, followed by stable form nucleation and growth. This transformation is caused by the difference in solubility of the two polymorphs and the resultant supersaturation level of the stable form I during crystallization. They found the process to be quite rapid and after 5 minutes, the suspended form II disappeared and chord length associated with growth of Form I increased. Solution concentration and supersaturation rapidly decreased, associated with the growth of form I. The results of the PAT and XRD measurements showed that among the 6 polymorphs of famoxadone, form I is the thermodynamically stable form and that the growth process of form I is the rate-determining step in the solvent-mediated polymorphic transformation.
Featured Article: Solvent and Supersaturation Control Polymorphism
ReactIR, ParticleTrack and EasyMax Support Solubility/Supersaturation Measurement and Particle Control
Teng Zhang, Yumin Liu, Shichao Du, Songgu Wu, Dandan Han, Shiyuan Liu, and Junbo Gong, “Polymorph Control by Investigating the Effects of Solvent and Supersaturation on Clopidogrel Hydrogen Sulfate in Reactive Crystallization”, Cryst. Growth Des, 2017, 17, 6123−6131.
The authors state that metastable polymorphic drugs, which frequently exhibit improved bioavailability, can be challenging to produce and maintain due to potential conversion to a more stable polymorph. The focus of their research was reactive crystallization of clopidogrel hydrogen sulfate (CHS) and investigating the two forms of the compound with respect to solvent and supersaturation levels. To determine the connection between solvent-solute interactions and consequential polymorphism, in-situ ATR-IR (ReactIR ) was used to continually monitor solute concentration and control supersaturation during crystallization and FBRM (ParticleTrack) was used to monitor particle numbers. The polymorphs were investigated in nine different solvents with different supersaturation and the reactions were performed in an automated laboratory reactor (EasyMax).
Data was presented for supersaturation and polymorphic formation of CHS in 2-propanol and 2-butanol to yield form II and form I, respectively, and ATR-FTIR and FBRM monitored the kinetics and real-time concentration of the CHS reactive crystallization. Different supersaturation levels were obtained by changing the amounts of clopidogrel and sulfuric acid. From this work, the authors determined that the nucleation induction period is the kinetic-determining step and supersaturation is the major driver for polymorphic formation of CHS reactive crystallization in the two different solvents. For the conditions and solvents used in these experiments, they found that the thermodynamically stable polymorph is obtained at s < 18, while the metastable form is formed at higher supersaturation level (s > 21).
Supersaturation in Recent Publications
- Barrett, M., McNamara, M., Hao, H., Barrett, P., & Glennon, B. (2010). Supersaturation tracking for the development, optimization and control of crystallization processes. Chemical Engineering Research and Design, 88(8), 1108–1119. https://doi.org/10.1016/j.cherd.2010.02.010
- Tacsi, K., Gyürkés, M., Csontos, I., Farkas, A., Borbás, E., Nagy, Z. K., Marosi, G., & Pataki, H. (2019). Polymorphic Concentration Control for Crystallization Using Raman and Attenuated Total Reflectance Ultraviolet Visible Spectroscopy. Crystal Growth & Design, 20(1), 73–86. https://doi.org/10.1021/acs.cgd.9b00539
- Szilagyi, B., Eren, A., Quon, J. L., Papageorgiou, C. D., & Nagy, Z. K. (2020). Application of Model-Free and Model-Based Quality-by-Control (QbC) for the Efficient Design of Pharmaceutical Crystallization Processes. Crystal Growth & Design, 20(6), 3979–3996. https://doi.org/10.1021/acs.cgd.0c00295
- Ezeanowi, N., Pajari, H., Laitinen, A., & Koiranen, T. (2020). Monitoring the Dynamics of a Continuous Sonicated Tubular Cooling Crystallizer. Crystal Growth & Design, 20(3), 1458–1466. https://doi.org/10.1021/acs.cgd.9b01103
- Milella, F., & Mazzotti, M. (2019). Estimation of the Growth and Dissolution Kinetics of Ammonium Bicarbonate in Aqueous Ammonia Solutions from Batch Crystallization Experiments. 2. The Effect of Sulfate Impurity. Crystal Growth & Design, 20(2), 948–963. https://doi.org/10.1021/acs.cgd.9b01315
- Ostergaard, I., Szilagyi, B., de Diego, H. L., Qu, H., & Nagy, Z. K. (2020). Polymorphic Control and Scale-Up Strategy for Antisolvent Crystallization Using Direct Nucleation Control. Crystal Growth & Design, 20(4), 2683–2697. https://doi.org/10.1021/acs.cgd.0c00101
- Sirota, E., Kwok, T., Varsolona, R. J., Whittaker, A., Andreani, T., Quirie, S., Margelefsky, E., & Lamberto, D. J. (2021). Crystallization Process Development for the Final Step of the Biocatalytic Synthesis of Islatravir: Comprehensive Crystal Engineering for a Low-Dose Drug. Organic Process Research & Development, 25(2), 308–317. https://doi.org/10.1021/acs.oprd.0c00520
- Nicoud, L., Licordari, F., & Myerson, A. S. (2018). Estimation of the Solubility of Metastable Polymorphs: A Critical Review. Crystal Growth & Design, 18(11), 7228–7237. https://doi.org/10.1021/acs.cgd.8b01200
- Nývlt, J. (1968). Kinetics of nucleation in solutions. Journal of Crystal Growth, 3–4, 377–383. https://doi.org/10.1016/0022-0248(68)90179-6
Applications
Applications For The Driving Force For Crystal Nucleation and Growth
Recrystallization is a technique used to purify solid compounds by dissolving them in a hot solvent and allowing the solution to cool. During this process, the compound forms pure crystals as the solvent cools, while impurities are excluded. The crystals are then collected, washed, and dried, resulting in a purified solid product. Recrystallization is an essential method for achieving high levels of purity in solid compounds.
Solubility curves are commonly used to illustrate the relationship between solubility, temperature, and solvent type. By plotting temperature vs. solubility, scientists can create the framework needed to develop the desired crystallization process. Once an appropriate solvent is chosen, the solubility curve becomes a critical tool for the development of an effective crystallization process.
Supersaturation occurs when a solution contains more solute than should be possible thermodynamically, given the conditions of the system. Supersaturation is considered a major driver for crystallization
In-process probe-based technologies are applied to track particle size and shape changes at full concentration with no dilution or extraction necessary. By tracking the rate and degree of change to particles and crystals in real time, the correct process parameters for crystallization performance can be optimized.
Seeding is one of the most critical steps in optimizing crystallization behavior. When designing a seeding strategy, parameters such as seed size, seed loading (mass), and seed addition temperature must be considered. These parameters are generally optimized based on process kinetics and the desired final particle properties, and must remain consistent during scale-up and technology transfer.
Liquid-Liquid phase separation, or oiling out, is an often difficult to detect particle mechanism that can occur during crystallization processes.
In an antisolvent crystallization, the solvent addition rate, addition location and mixing impact local supersaturation in a vessel or pipeline. Scientists and engineers modify crystal size and count by adjusting antisolvent addition protocol and the level of supersaturation.
Crystallization kinetics are characterized in terms of two dominant processes, nucleation kinetics and growth kinetics, occurring during crystallization from solution. Nucleation kinetics describe the rate of formation of a stable nuclei. Growth kinetics define the rate at which a stable nuclei grows to a macroscopic crystal. Advanced techniques offer temperature control to modify supersaturation and crystal size and shape.
Changing the scale or mixing conditions in a crystallizer can directly impact the kinetics of the crystallization process and the final crystal size. Heat and mass transfer effects are important to consider for cooling and antisolvent systems respectively, where temperature or concentration gradients can produce inhomogeneity in the prevailing level of supersaturation.
Crystal polymorphism describes the ability of one chemical compound to crystallize in multiple unit cell configurations, which often show different physical properties.
Protein crystallization is the act and method of creating structured, ordered lattices for often-complex macromolecules.
Lactose crystallization is an industrial practice to separate lactose from whey solutions via controlled crystallization.
The MSMPR (Mixed Suspension Mixed Product Removal) crystallizer is a type of crystallizer used in industrial processes to produce high-purity crystals.
A well-designed batch crystallization process is one that can be scaled successfully to production scale - giving the desired crystal size distribution, yield, form and purity. Batch crystallization optimization requires maintaining adequate control of the crystallizer temperature (or solvent composition).
Continuous crystallization is made possible by advances in process modeling and crystallizer design, which leverage the ability to control crystal size distribution in real time by directly monitoring the crystal population.
Recrystallization is a technique used to purify solid compounds by dissolving them in a hot solvent and allowing the solution to cool. During this process, the compound forms pure crystals as the solvent cools, while impurities are excluded. The crystals are then collected, washed, and dried, resulting in a purified solid product. Recrystallization is an essential method for achieving high levels of purity in solid compounds.
Solubility curves are commonly used to illustrate the relationship between solubility, temperature, and solvent type. By plotting temperature vs. solubility, scientists can create the framework needed to develop the desired crystallization process. Once an appropriate solvent is chosen, the solubility curve becomes a critical tool for the development of an effective crystallization process.
In-process probe-based technologies are applied to track particle size and shape changes at full concentration with no dilution or extraction necessary. By tracking the rate and degree of change to particles and crystals in real time, the correct process parameters for crystallization performance can be optimized.
Seeding is one of the most critical steps in optimizing crystallization behavior. When designing a seeding strategy, parameters such as seed size, seed loading (mass), and seed addition temperature must be considered. These parameters are generally optimized based on process kinetics and the desired final particle properties, and must remain consistent during scale-up and technology transfer.
Crystallization kinetics are characterized in terms of two dominant processes, nucleation kinetics and growth kinetics, occurring during crystallization from solution. Nucleation kinetics describe the rate of formation of a stable nuclei. Growth kinetics define the rate at which a stable nuclei grows to a macroscopic crystal. Advanced techniques offer temperature control to modify supersaturation and crystal size and shape.
Changing the scale or mixing conditions in a crystallizer can directly impact the kinetics of the crystallization process and the final crystal size. Heat and mass transfer effects are important to consider for cooling and antisolvent systems respectively, where temperature or concentration gradients can produce inhomogeneity in the prevailing level of supersaturation.
A well-designed batch crystallization process is one that can be scaled successfully to production scale - giving the desired crystal size distribution, yield, form and purity. Batch crystallization optimization requires maintaining adequate control of the crystallizer temperature (or solvent composition).

















