Crystallization Determination Thermal Analysis Instruments
Investigate Crystallization Properties
Thermal analysis is often used to determine the crystallization behavior of materials. Crystallization refers to the formation of crystal structures in a material, such as polymers or other organic and inorganic materials. Crystallization, as well as polymorphism, analysis can be performed with differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), thermomechanical analysis (TMA), and dynamic mechanical analysis (DMA). Hot stage microscopy or other optical techniques allow the visualization of crystal formation.
Advantages
Modular Design
Modularity is at the core of METTLER TOLEDO thermal analyzers, enabling us to offer tailor-made solutions for almost all academic and industrial applications. Should requirements change after installation, the instrument can be upgraded as needed.
Reliable Automation
Our TGA and DSC systems support fully automated workflows – from sample loading to result analysis and report generation. Up to 34 samples can be processed by our robust, factory endurance-tested sample robot.
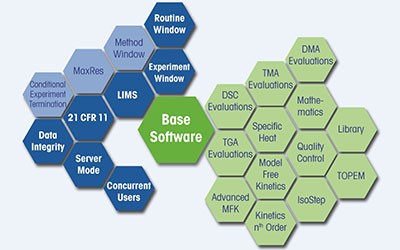
One Software for All Thermal Analysis Instruments
STARe is the most complete and comprehensive thermal analysis software, providing unrivalled flexibility, unlimited evaluation possibilities, and the technical controls to support compliance.

Innovative Sensor Technology
Put your trust in METTLER TOLEDO’s world-leading sensor technology: Whether you purchase a DSC, TGA, TMA or DMA instrument, we guarantee exceptional sensitivity and highly accurate measurement results.
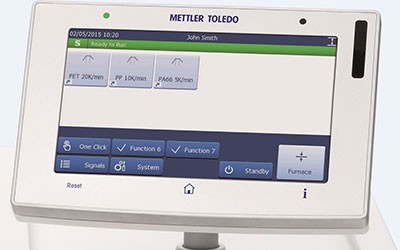
Simple Operation
STARe functionality is readily accessible from the software's intuitive ribbon interface. Standard features, such as OneClick™ and multiple curve handling, as well as options like Quality Control and Reference Library, simplify daily lab work.

High-Level Competence & Support
Our global network of application specialists gives you access to the latest application know-how. Dedicated application specialists help to ensure that you obtain the most accurate thermal analysis results.
Explore Our Services - Tailored to Fit Your Equipment
According to the International Confederation for Thermal Analysis and Calorimetry (ICTAC), thermal analysis is group of techniques in which a physical property of a substance is measured as a function of temperature while the substance is subjected to a controlled temperature program.
Support & Repair

Training & Consulting

FAQs
How is differential scanning calorimetry (DSC) used for crystallization determination?
Differential scanning calorimetry is a thermal analysis technique that measures the heat flow into or out of a sample as it is heated or cooled. Differential scanning calorimetry can be used to determine the crystallization temperature and enthalpy of crystallization of a material. A DSC provides important information about a material's thermal behavior and crystal formation.
How does thermogravimetric analysis (TGA) help in determining the crystallization behavior of materials?
Thermogravimetric analysis is a technique that measures the change in weight of a sample as it is heated or cooled. When a TGA is equipped with thermocouples, it can be simultaneously used to study the crystallization behavior and decomposition of a material. A TGA/DSC can be used at temperatures up to 1600°C, therefore, high temperature crystallization behavior can also be measured.
How is thermomechanical analysis (TMA) used in studying the crystallization behavior of materials?
TMA measures the dimensional changes of a material as a function of temperature, time, or force. During crystal formation, materials may undergo a change in volume due to the formation of crystal structures. TMA can detect these changes in volume and provide information about the degree and rate of crystallization.
How is dynamic mechanical analysis (DMA) used in studying the crystallization behavior of materials?
Dynamic mechanical analysis is a technique that measures the mechanical properties of a material as a function of temperature, time, or frequency. Dynamic mechanical analysis is used to study the mechanical behavior of a material during crystallization and crystal formation, such as changes in stiffness, modulus, or damping.
How is Flash DSC (FDSC) used to determine the crystallization behavior of materials?
In Flash DSC, a small amount of sample is heated or cooled at ultra-fast rates. Therefore, a material can be locked in a particular state without the possibility of rearrangement. Flash DSC is commonly used to determine isothermal crystallization behavior.
How is thermo-optical analysis (TOA) used in studying the crystallization behavior of materials?
Optical techniques, such as hot-stage microscopy, are a useful tool for determining the behavior of materials as they go through a crystallization process. As a material starts to crystalize, the nuclei will become visible; therefore, qualitative results can be obtained. This is especially helpful for materials that exhibit polymorphism.
What type of materials are studied by thermal analysis?
The crystallization behavior of polymers, metals and alloys, pharmaceuticals, and food are studied by thermal analysis.
What is polymorphism?
Polymorphism refers to the ability of a substance or material to exist in multiple crystal structures or forms, known as polymorphs. Polymorphs have the same chemical composition but differ in their physical properties, such as melting point, density, and solubility, due to differences in their crystal structure. Polymorphism is important as the properties of a specific polymorph can affect the efficacy and stability of a drug, or the properties of a material.