EasyMax 102 Advanced and HFCal
Calorimetry for Safety Screening.The EasyMax 102 HFCal: a reaction calorimeter designed for fast and efficient safety screening and characterization of chemical reactions at small scale.
Uncover Potential Safety Issues
Non-scalable conditions such as accumulation, bad mixing, or dosing issues can easily be identified with EasyMax 102 HFCal.
True Heat Flow Is Important
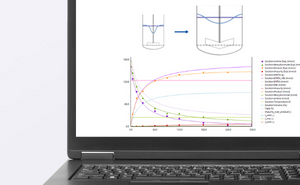
Unlike conventional heat flow calorimetry, EasyMax HFCal determines the true heat as released by the chemical reaction.
Process Development - Fast and Reliable
Continuous monitoring of the reaction progress, the heat release, and possible accumulation makes process development faster and more predictable.
Material No.: 511617111

| Weight | 18 kg |
| Operating Volume | 40 mL – 100 mL |
| Dimensions (HxWxD) | 28 cm x 33 cm x 36 cm |
| Temperature Range | -40 °C – 180 °C |
| Back / Front light | Yes/No |
| Precision Heat Transfer | Typically +/- 4% |
| Accuracy and Precision Specific Heat | Typically +/-12% |
| Accuracy Heat Flow | Based on comparison of qr_hf with qc resp. ∫qr_hf with ∫ qc Isothermal conditions: ± 3 % to 5 % Non-isothermal conditions: ± 5 % to 10% |
| Calorimetry Type | Heat Flow |
| Material | EasyMax 102 Advanced (51161711) and Upgrade Kit EasyMax HFCal (30090576) |
| Temperature Modes | Jacket control, reaction mixture control, distillation control, crystallization control |
| Heating / Cooling | Solid State Thermostat |
| Power Requirements | 100 – 240 VAC, 50/60Hz |
Data Analysis - Efficient and Accurate
The EasyMax 102 HFCal collects and stores a wealth of information during an experiment. iControl software automatically calculates heat transfer data, specific heat of the reaction mass, heat flow and reaction enthalpies, and it presents all data in a report.
Calorimetry from Screening to Production
The EasyMax 102 HFCal provides safety information early in development at small scale with minimal effort. Heat data together with chemical and kinetics information are the basis for additional investigation to ensure safe scale-up of processes.
Integration with Analytical Probes
The seamless integration of heat data with online analytical techniques and other process data significantly enhances understanding of the course of the reaction, its potential risk, and the formation of by-products to allow users to draw better conclusions.