Titrators
A Complete Range of Titrators and Accessories That Cover Your Titration Applications
A titrator determines the amount of a substance, or analyte, which is dissolved in a sample. Through a controlled addition of reagent in a known volume, the chemical reaction is monitored either by color change with a photometric sensor or with a suitable pH, redox, conductivity or surfactant sensor. A Karl Fischer titrator determines the amount of water in a sample in a range from 0.001% with coulometric Karl Fischer to 100% water content with volumetric Karl Fischer titration.
Advantages of METTLER TOLEDO’s Titrators
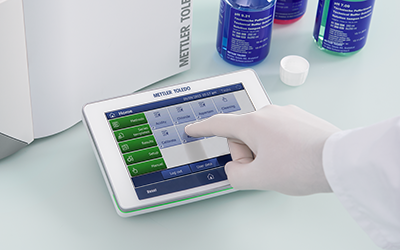
Simple Operation
The One Click® interface runs simple or complex methods and analyses with a single tap. Customize your personal shortcuts and start your workflows quickly and easily.

Dedicated to Your Needs
From simple and affordable titrators to expandable, modular, fully automated solutions with data management; we have a titrator to suit your laboratory’s needs.

Future Ready
Do you know what you need in 5 years’ time? Thanks to a modular concept, our titrators can be easily expanded with Karl Fischer titration, high throughput autosamplers, liquid handing and dosing workflows, and LabX™ software to cover your future requirements.

Seamless Workflow with Automation
Save time, reduce costs and improve operational safety with dedicated automation solutions. Upgrade from simple sampling pumps and liquid dispensing to high throughput autosamplers.

Increase Productivity
Decrease your time to results and reporting by seamlessly integrating your titration system with your electronic workflows. Our smart accessories wirelessly transfer data from balances and reagents / chemicals to save you time and reduce errors.
Solid Compliance
Meet compliance and data integrity standards easily and efficiently with central user management, electronic signatures and an audit trail. Full compliance support for 21 CFR part 11 and EU Annex 11 with the LabX™ software.
Free Application Downloads
More than 1000 ready-made titration applications are available for download. These proven and well-tested titration applications ensure you quickly achieve accurate results. Read more
Explore our Services - Tailored to Fit your Equipment
We support and service your measurement equipment through its entire lifecycle, from installation to preventive maintenance and calibration to equipment repair.
Support & Repair

FAQs
How does an autotitrator work?
Automated titrators from METTLER TOLEDO follow a defined sequence of operations. This sequence is basically the same for all different models and brands. It is performed and repeated several times until the endpoint or the equivalence point of the titration reaction is reached (titration cycle). The titration cycle consists mainly of 4 steps:
- Titrant addition
- Titration reaction
- Signal acquisition
- Evaluation
Each step has different specific parameters (e.g. increment size), which have to be defined according to the specific titration application. More complex applications require more steps, e.g. dispensing of an additional reagent for back titrations, dilution, adjusting of the pH value. These steps and the corresponding parameters are resumed in a titration method.
How often should I clean a titrator?
Depending on the frequency of usage, you should clean the titrator equipment as burette cylinder, piston, valve and tubing relatively often. It is important to use high quality ethanol for the cleaning procedure.
- Depending on the contamination caused by the standard, rinse cylinder, valve and tubes with deionized water then with ethanol
- Dry the parts with oil-free compressed air
Why, when I perform an equivalence point titration using an automatic titrator, do I get a different result compared to when I titrate manually using a color indicator?
This discrepancy in results is primarily noticeable when performing acid/base titrations using one of the pH indicators. The first reason for this is that these pH indicators change color over a pH range rather than at a fixed value. The actual point at which the color change occurs is very much sample dependant and may not coincide with the chemical equivalence point. This can result in a small discrepancy in result which is easily nullified by standardizing the titrant using a similar method as is used for samples.
The second reason for this difference is primarily one of the sensitivity of the human eye to color change. While a color change may have already started to occur, the human eye has still not detected any change. This can be demonstrated by using a photometric sensor such as the METTLER TOLEDO DP5 Phototrode™. Using one of these sensors there is a clear change in light transmittance long before the human eye detects any color change. In the typical acid/base titration using potentiometric indication with a pH sensor, the sharp change in signal occurs at the first trace of excess acid (or base) and is therefore a more true indication of the end point.
What electrode should I use for non-aqueous titrations on my titrator?
Generally, there are three main electrode or sensor problems when performing a non-aqueous titration. The first is the problem of having an aqueous electrolyte with a non-aqueous solvent. Replacing the electrolyte in the electrode easily solves this. The second problem relates to the fact that the sample is non-conductive, resulting in a poor electrical circuit between measuring and reference half-cells or parts of the electrode if combined. This results in a noisy signal, particularly when using a sensor with a standard ceramic junction in the reference. A partial solution to this problem is to use a sensor with a sleeved junction, such as the DGi113 sensor. This sensor has LiCl in ethanol as the standard electrolyte and, rather than a ceramic junction, has a polymer sleeve resulting in a larger contact area between working and reference parts and therefore lower noise.
The third problem is not a problem of the electrode itself, but rather the handling of the sensor. In order for a glass (pH) sensor to function correctly, it is necessary that the glass membrane (bulb of electrode) is hydrated. This is achieved by conditioning the electrode in deionized water. During the non-aqueous titration this membrane is gradually dehydrated reducing the response of the electrode. To prevent this or rectify this problem the electrode should be regularly reconditioned by soaking in water.
How can one export data from METTLER TOLEDO titrators?
The traditional way to keep titration’s results is by printing them, either on a USB -P25 tape compact printer or on an A4 USB printer. However, METTLER TOLEDO titrators offer other possibilities like direct data export and pdf or xml reports. Furthermore, the results can be saved on a USB stick, sent to a connected PC or to a remote network folder. Physical printers (A4 printers or compact printers) or virtual printers (RS232 or USB data export, PDF/XML file writers) are triggered by the “Record” method function inside a method. The “Record” method function can be customized. In parallel, the titrator automatically generates a CSV file after each sample using a standard template and saves them to a USB stick or a network folder. Results can be sent at the same time to a printer (physical or virtual) and as CSV.
What is the difference between volumetric and coulometric Karl Fischer Titrator?
The titrant can either be added directly to the sample by a burette (volumetry) or generated electrochemically in the titration cell (coulometry). The coulometric titration is mainly employed for the water determination according to Karl Fischer when the content is very low, e.g. smaller than 50-100 ppm (0.005-0.01%).
When should I use a Karl Fischer cell with or without diaphragm for METTLER TOLEDO titrators?
The C20S and C30S are available with two different coulometer cells – with or without a diaphragm. For most applications, we recommend the cell without the diaphragm because it is almost maintenance-free. Due to its innovative design, this diaphragm-free cell from METTLER TOLEDO can even be used for the determination of water in oils. The version of the cell with a diaphragm is recommended for applications such as the determination of water in substances containing ketones. It is also recommended if the best possible accuracy is required.
How often do I need to replace the solvent in the titration beaker of my Karl Fischer titrator?
The first and most obvious answer to this question is that the solvent should be replaced as soon as the sample no longer dissolves. This, however, is only one of the reasons for changing the solvent. A second less obvious reason applies in the case of two component reagent where the titrant contains iodine and the solvent contains all the other components necessary for the Karl Fischer reaction. One of these other components is sulfur dioxide and this can become depleted long before the dissolving capacity of the solvent is exceeded. As a general rule the solvent in these two component systems has an approximate water capacity of 7mg of water per mL of solvent. This means that in theory 40mL of solvent can accommodate 280mg of water before the solvent need be changed. As the typical titrant has a concentration of 5mg/mL, 280mg of water would require 56mL of titrant.
How do I know when to replace the molecular sieve in the drying tubes on my Karl Fischer titrator?
The most practical solution to this question is to add some blue silica gel to the top of the drying tube to serve as an indicator. As soon as the first trace of pink appears in this gel layer, it is time to change or regenerate the molecular sieve. Naturally, an increase in background drift can also indicate that it is time to replace the molecular sieve.
How do I go about validating a method on my automatic titrator?
When validating a titrator method one needs to check things like accuracy, precision, reproducibility, linearity, systematic errors, robustness, ruggedness and limits of determination. For detailed recommendations on how to go about this validation please refer to our sections on quality control and validation or refer to the METTLER TOLEDO applications brochure 16 - Validation of Titration methods.